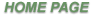Protection of DNA
against thermodegradation by salts at temperatures typical for
hyperthermophiles
Evelyne Marguet and Patrick Forterre
Institut de génétique et Microbiologie,
Université Paris-Sud, 91405 Orsay Cedex, France.
DNA molecules are exposed at very high temperatures (80-110°C) for long period in
hyperthermophiles and for short periods in biotechnological procedures involving thermophilic
enzymes. These temperature stress can potentially induces various chemical modifications, the
main one being depurination followed by cleavage of the nearby phosphodiester bond. Heat-
induced DNA damages should be repaired in hyperthermophiles, but it is not clear to what extent
their repair systems have to be be efficient. On the other hand, DNA damages induced by high
temperature in vitro cannot be repaired and might lead to various types of error cascades.
Most studies on DNA thermodegradation in vitro have been initially performed using linear
double-stranded DNA at temperatures below the Tm (usually 70°C-80°C). We have recently
initiated similar investigations at temperatures more typical for hyperthermophiles, i.e. from 90 to
110°C, using supercoiled plasmids. Plasmids are much more resistant to thermodenaturation than
linear DNA, since the topological links between the two strands cannot be eliminated, as long as
the they are covalently closed. We have shown that a bacterial plasmid is indeed resistant to
denaturation, at least up to 107°C; but is rapidly degraded at such temperature (Marguet and
Forterre, 1994). Although hyperthermophiles possess an enzyme, reverse gyrase, which
produces positively supercoiled DNA (for review, see Forterre et al., 1996), positively
supercoiled plasmids are no more resistant to thermodegradation than negatively supercoiled
ones. However, subsequent thermodegradation was reduced in the presence of physiological
concentrations of either monovalent or divalent salts (Marguet and Forterre, 1994).
Protection of DNA against thermodegradation by salts could be relevant for
hyperthermophilic life, since some hyperthermophiles exhibit very high intracellular salt
concentrations. They can be also significant for DNA manipulation in vitro . Here, we report
further experiments on this problem. In particular, we have compared the effect of KCl and
MgCl2 on double-stranded and single-stranded DNA, and on the two steps of DNA degradation,
depurination and subsequent cleavage. We show that KCl and MgCl2 protect both single and
double-stranded DNA against thermodegradation via the inhibition of depurination. This
indicates that the protection effect of salt on the thermodegradation of double-stranded DNA is
not mediated by the stabilization of the double-helix, but by a direct protection of the N-
glycosidic bond at high temperature. Our results also shown that formation of apurinic sites is
not immediatly followed by DNA cleavage, even at hyperthermophilic temperatures. They
suggest that the number and nature of heat-induced DNA lesions which have to be repaired might
be quite different from one hyperthermophile to another, depending of their intracellular salt
concentration. We plan now to study the effect of compatible solute on DNA stability at high
temperature in collaboration with our EC partners.
References:
Marguet, E. and Forterre, P.
DNA stability at temperatures typical for hyperthermophiles.
Nucl. Acid Res. 22, 1681-1686 (1994)
Forterre, P., Bergerat, A. and Lopez-Garcia, P.
The unique DNA topology and DNA topoisomerases of hyperthermophilic archaea.
FEMS Microbiol. Rev. 18, 237-248 (1996)
|

To see an overview of the poster follow
this link